Biomed Industries Presents Breakthrough Phase 2 Results of NA-931, an Oral Quadruple Agonist for Obesity , at ADA 2025
Biomed Industries, Inc. Presents Breakthrough Phase 2 Results of NA-931, a First-in-Class Oral Quadruple Agonist for the Treatment of Obesity , at ADA 2025
NA-931's Phase 2 results highlight its potential as a first-in-class oral quadruple receptor agonist. With excellent safety and efficacy, we have advanced it to Phase 3 as a novel obesity treatment.”
SAN JOSE, CA, UNITED STATES, June 23, 2025 /EINPresswire.com/ -- Biomed Industries, Inc. (Biomed) today announced that its CEO Dr. Lloyd Tran presented topline results from the Phase 2 clinical trial of NA-931, a novel oral quadruple receptor agonist for obesity, at the 85th American Diabetes Association (ADA) Scientific Sessions, held June 20–23, 2025, in Chicago, IL.— Dr. Lloyd L. Tran, CEO of Biomed
The presentation, titled “NA-931, A Novel Quadruple IGF-1, GLP-1, GIP, and Glucagon Receptor Agonist Reduces Body Weight Without Muscle Loss,” included summaries of the mechanism of action, Pharmacokinetics studies, randomized placebo-controlled Phase 1 and Phase 2 study.
Mechanism of Action:
NA-931 is a small molecule, which is a quadruple receptor agonist of the insulin-like growth hormone- (IGF-1), glucagon-like peptide 1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP) and glucagon receptor agonist. NA-931 is the first-in-class that is administered orally.
Pharmacokinetics
Pharmacokinetic data supports a once-daily dosing regimen for NA-931. Blood levels of the drug remained consistent regardless of fasting or after a high-fat meal, suggesting that NA-931 can be taken without regard to meal timing, offering greater flexibility for patients. </p>
PHASE 2 RESULTS:
This is a phase 2, 13-week randomized, double-blind, placebo-controlled, parallel arm study that will evaluate the safety, tolerability, weight loss efficacy of NA-931 in adults who are obese (BMI ≥30 kg/m2) or who are overweight (BMI ≥27 kg/m2) with at least one weight-related co-morbid condition. Number of enrolled subjects was 125 participants.
Body Weight Reductions:
The 13-week MAD study showed NA-931 demonstrated dose-dependent reductions in mean body weight from baseline, up to 13.8 % at 150 mg daily dosage, or 12.4% % relative to placebo.
Safety and Tolerability:
Among subjects receiving NA-931, treatment emergent adverse events (TEAEs) were reported have been insignificant or mild. All observed gastrointestinal (GI) adverse events have been reported as insignificant or mild. Mild nausea and vomiting were reported as mild in 7.3% in NA-931-treated subjects. Diarrhea was reported in 6.3% of subjects receiving NA-931. No muscle loss was observed. No clinically meaningful differences were reported for GI-related adverse events among subjects treated with NA-931 compared with placebo.
Unlike many existing therapies, NA-931 not only promotes weight loss but also preserves muscle mass, while showing a lower incidence of adverse effects typically associated with current obesity treatments.
“The Phase 2 results of NA-931 highlight its potential as a first-in-class oral quadruple receptor agonist for weight loss, with excellent safety and efficacy,” said Dr. Lloyd L. Tran, CEO of Biomed Industries. “We are excited to advance NA-931 to Phase 3 trials, aiming to provide a more comprehensive and well-tolerated treatment option for obesity.”
PRESS CONFERENCE:
Biomed will host a press conference on Monday, June 23, 2025 at 10:30 AM CT during the ADA Conference located at the McCormick Place (exact location TBD0
👉 Register here: https://form.jotform.com/251658034520149
THE URGENT NEED FOR EFFECTIVE AND SAFE OBESITY TREATMENT
Obesity remains a critical global health challenge, contributing to comorbidities such as type 2 diabetes, cardiovascular disease, liver disease, and chronic kidney disease. More than 650 million people worldwide are affected by obesity, and this figure is expected to surpass 50% of the global population by 2035. Current treatments often target limited aspects of the condition, underscoring the need for more comprehensive therapies like NA-931.
ABOUT NA-931:
NA-931 is a first-in-class, orally active, small-molecule quadruple receptor agonist that simultaneously targets IGF-1, GLP-1, GIP, and glucagon receptors. This multi-pathway approach restores metabolic balance and induces clinically meaningful weight loss—without muscle loss or severe side effects. In Phase 1 trials, NA-931 demonstrated potential benefits for both weight reduction and glycemic control in individuals with type 2 diabetes.
Biomed recently completed a Phase 2, randomized, double-blind, placebo-controlled, 13-week study in patients with obesity (BMI ≥30) or overweight (BMI ≥27) with at least one weight-related comorbidity. Topline results will be announced on June 20, 2025, at ADA 2025. (ClinicalTrials.gov ID NCT06564753)
ABOUT BIOMED INDUSTRIES, INC.
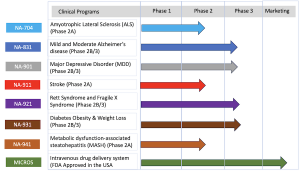
Biomed Industries, Inc. is a pioneering biopharmaceutical company committed to developing novel therapeutics that address unmet medical needs. Its innovative research platform has produced treatments for conditions including Alzheimer’s disease, ALS, Traumatic Brain Injury, Major Depressive Disorder, Diabetes, Obesity, MASH, Stroke, and rare diseases like Rett Syndrome.
For more information, please contact:
Biomed Industries, Inc.
info@biomedind.com
https://.www.biomedind.com
Michael Willis
Biomed Industries, Inc.
+1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.